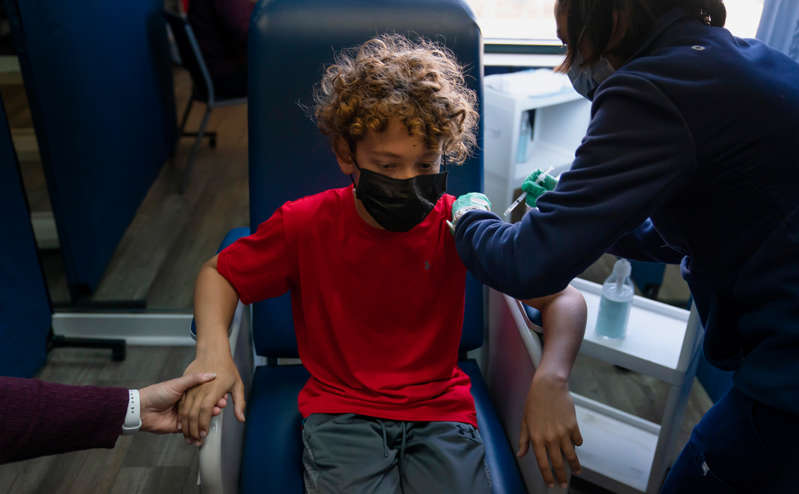
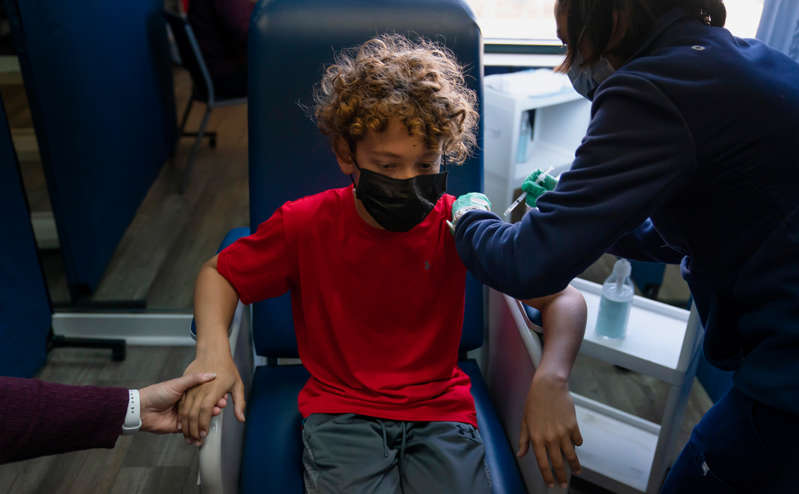
The Pfizer and BioNTech coronavirus vaccine has successfully passed the second phase of trials in children aged 5 to 11, the company said.
“For participants aged 5 to 11, the vaccine was found to be safe, well tolerated and showed a sustained neutralizing antibody response,” Pfizer said in a statement.
Children under 12 years old who took part in the trials were injected with two doses of 10 mcg of the drug (people from 12 years old are injected with 30 mcg) with an interval of three weeks, the company said. According to the company, for children of this age, a dosage of 10 mcg is optimal from the point of view of safety and the appearance of immunity.
Pfizer said the children showed a “strong” immune response after the second dose, with side effects comparable to those seen in participants aged 16 to 25.
Up to 4,500 minors from the United States, Finland, Poland and Spain of different ages – from six months to 11 years old – should take part in all three phases of the Pfizer vaccine trials on children. The company divided the children for research into three age groups: the first included children aged from six months to two years, the second – from two to five years, and the third – from five to 11 years.
Children under the age of five received an even lower dose compared to adults or slightly older children, they were injected with 3 μg during each of the two injections.
Pfizer said it intends to send the research results to the US and EU regulators as soon as possible. Pfizer plans to get the first data from studies in other children's groups (six to two years and two to five years) no earlier than the fourth quarter of this year.
From the start of the pandemic to September 9, the coronavirus was detected in about 5.3 million American children, the company said on Twitter. Moreover, about 500 thousand children fell ill within two weeks before this date, Pfizer said.
In the United States, from the beginning of May, vaccination of children aged 12 years and older has been allowed. The FDA noted that the known benefits of vaccinating children from this age outweighed the potential risks.
At the end of August, Russian Health Minister Mikhail Murashko said that children became more likely to get coronavirus due to the “aggressiveness of the pathogen.” According to him, parents can protect children if they get themselves vaccinated against COVID-19.
In Russia, children are not yet vaccinated against coronavirus. Since the beginning of July, Moscow has been testing Sputnik V on children and adolescents aged 12 to 17 years. They were given the same vaccine as adults, but at a lower dosage. The authorities plan to conduct the study during the year.

