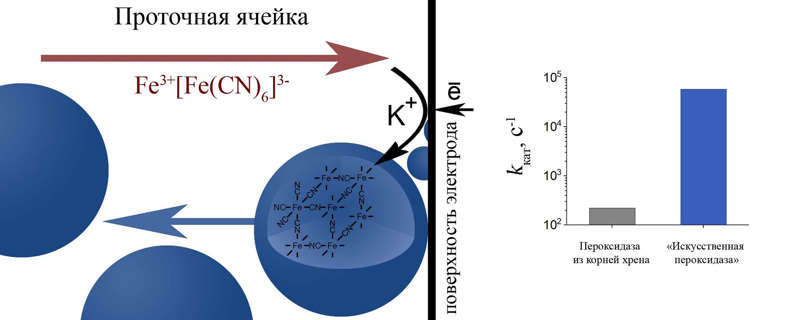
The essence of the new approach to the creation of nanozymes and the results of the study of catalytic activity.
Chemists of Lomonosov Moscow State University have proposed a new method for producing nanozymes with ultrahigh peroxidase activity based on Prussian blue. These nanoparticles accelerate the conversion of hydrogen peroxide to water. Such a reaction can serve as the basis for the creation of sensors and biosensors for monitoring the concentration of metabolic products and will help to track violations of oxygen metabolism in cells. Scientists synthesized nanozymes were 200 times more effective than a natural enzyme that performs the same function. The proposed electrochemical approach can be adapted to obtain functional nanomaterials based on conductive and electroactive polymers. The results of the work, supported by a grant from the Russian Science Foundation (RSF), are published on the pages of Dalton Transactions.
Hydrogen peroxide (H2O2) is a chemically very active compound that is used almost everywhere. With its help, foamy materials are produced, discolored tissues and hair, disinfected not only wounds, but also sewage. In this case, H2O2 interacts with cells and destroys them, which is traumatic for large organisms and destructive for microscopic ones. Hydrogen peroxide not only enters living beings from the outside, but is also formed during various pathological processes, for example, violations of oxygen metabolism, so it is important to be able to accurately determine its content in samples from patients and in the environment.
For accurate detection of hydrogen peroxide, biosensor devices based on peroxidase, a natural enzyme that protects against the destructive activity of H2O2 in living organisms, can be used. In the course of a chemical reduction reaction, it turns it into safe water by transferring electrons from some other compound to it. To determine the content of peroxide in analytics, such a donor substance is molecules that are uncolored in their usual form, but after the loss of electrons acquire color – by its saturation, one can determine the concentration of H2O2. “Natural enzymes are difficult to beat, but getting them directly from cells can be expensive and difficult, and it is also important to purify the product well and not lose its activity outside the living system. Therefore, in 1965, particles with similar properties – artificial enzymes – appeared as an alternative. Despite several decades of research in this area, the optimal solution for biosensorics was proposed only in 2004 by Professor Skrimin's group. Scientists have demonstrated the high enzymatic activity of inorganic nanoparticles – nanozymes. But here we are faced with the fact that the properties of the obtained substances strongly depend on the synthesis conditions, ”says Maria Komkova, PhD in Chemistry, senior researcher at the Research Laboratory of Electrochemical Methods, Faculty of Chemistry, Lomonosov Moscow State University.
Researchers at the Faculty of Chemistry of Lomonosov Moscow State University (Moscow) have proposed a new method for the synthesis of nanozymes based on Prussian blue, an available blue pigment that is extremely sensitive to hydrogen peroxide. The essence of the approach lies in the fact that an aqueous solution of salts necessary for the production of artificial enzymes is continuously passed through a special flow cell – a small reservoir with electrodes on which electrochemical synthesis takes place. As a result, the electrodes are covered with tiny particles of Prussian blue, which are washed off by the stream and removed from the cell with it. In other words, the scientists managed to adapt approaches to the nanostructuring of the material on the electrode surface and obtain suspensions of individual nanoparticles, which also demonstrated excellent catalytic properties.
“We applied enzymatic kinetic approaches to investigating the activity of our nanozymes. It turned out that the particles produced by the new method are 200 times more efficient than natural peroxidase in terms of catalysis efficiency. We hope that our method will help change modern bioanalytics. These nanozymes are stable and very active, and their size can be changed by choosing the component composition of the solution for synthesis and the applied voltage. This makes it possible to use both individual nanostructures and coatings based on them in biosensor practice. As a result, nanozymes can be used for cell research and for the industrial production of biosensors. In the future, we plan to test our nanoparticles to reduce the concentration of reactive oxygen species directly inside the cells, ”sums up Maria Komkova.
Read also:
Neuroscientists have proposed a new concept for the organization of the brain at the cellular level
Mesolithic sites explored in Murom district
A new season of the intellectual online game League of Knowledge “Natural Intelligence” has started
Skolkovo launched a hub for EdTech companies
10 billion rubles will be invested in a new venture fund

