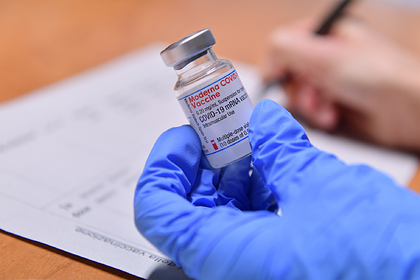
The chief epidemiologist of the American state of California, Erica Pan, issued a recommendation in which she proposed to temporarily stop using the Moderna coronavirus vaccine due to the increased likelihood of allergies from the drug in one of the supplies.
“A higher than usual number of possible allergic reactions was recorded from a particular batch of Moderna vaccine at one of the vaccination clinics. Less than 10 patients required medical attention within 24 hours, ”Pan said.
The statement was released because the medic wanted to convey to all the agencies distributing the vaccine that the drug from this supply should not be used until the investigation of its peculiarities is completed. The batch reportedly contained more than 330,000 doses distributed to 287 vaccination sites.
An acute allergy to the Moderna vaccine was first identified in the United States at the end of December 2020. She was recognized as an isolated case.
There are currently several COVID-19 vaccines in the world. Thus, two vaccines are registered in Russia: Sputnik V and EpiVacCorona. On December 5, Moscow started vaccination against coronavirus with Sputnik V. Pfizer / BioNTech has a COVID-19 vaccine registered in the United States and another developed by Moderna. Also, the British pharmaceutical company AstraZeneca announced the beginning of clinical trials for a combination of its AZD1222 vaccine.

